IVD Blood Test for Glut1DS Diagnosis

METAglut1™ is the solution to a major unmet need to aid diagnose children & adults
thanks to a non invasive, fast and affordable test for a
damaging disease that can be treatable.
Want to receive updates and news about METAglut1™:
The Glut1 Deficiency Syndrome
Glut1DS, or De Vivo disease,
is a rare neurometabolic syndrome caused by
a defect in the glucose transporter type 1 (Glut1),
resulting in a cerebral energy deficit.
The disease is linked to mutations in the SLC2A1
gene coding for the GLUT1 protein, mostly
heterozygous dominant and de novo.

MEDICAL WANDERING
> 95 %
of patients are currently
NOT diagnosed
Mean age at diagnosis
is 8 years old
Puzzled by
neurological symtoms?
- Neurodevelopmental disorders
(intellectual disability or specific mixed neurodevelopmental disorder) - Epilepsy
(childhood onset, drug resistance, seizures associated with fasting…) - Movement disorders
(paroxysmal or permanent) - Abnormal eye movements
- DEE
(developmental and epileptic encephalopathy)

Think
Glut1DS…
Glut1DS is characterized by a
wide phenotypic spectrum
with a combination and severity of symptoms that greatly vary from one patient to another.

Early Detection is Key


Treatable with a ketogenic diet (KD)

Late diagnosis leads to
irreversible brain damage
“It is essential to diagnose as early as possible to allow prompt compensation,
through the Ketogenic Diet, for the brain’s lack of fuel…”
“Early identification of children with the disease is important in order to avoid submitting them to possibly ineffective or potentially detrimental treatments with anticonvulsants”
De Giorgis, P. Veggiotti / Seizure 22 (2013) 803–811
– GLUT1 deficiency syndrome 2013: Current state of the art.

METAglut1™
A direct quantification of GLUT1
on erythrocytes
- On a simple blood draw
- No need for fasting
- Outpatient or inpatient settings
- Quick turnaround time (24-72h)
- Great performance*
Equivalent to glycorrhachia
Sensitivity »80%
Specificity >99%
PPV =90%
NPV =97%
*Results published in
Mochel F. et al. Prospective Multicenter Validation of
a Simple Blood Test for the Diagnosis of Glut1
Deficiency Syndrome. Neurology. 2023 Jun
6;100(23):e2360-e2373
METAglut1™ used as a first line simple test
to search for Glut1DS patients**
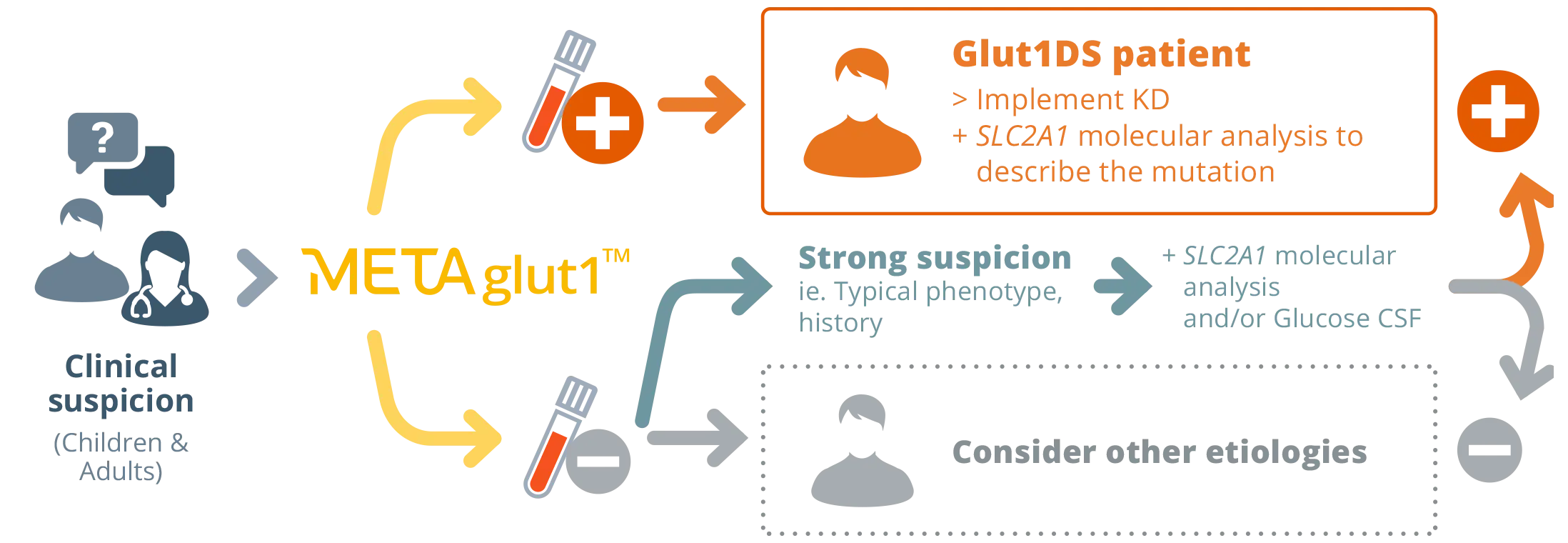
** Strategy published in the journal Neurology* and recommended by the HAS: Avis n°2023.0011/AC/SEAP du 30 mars 2023 du collège de la Haute Autorité de santé relatif à l’inscription sur la liste des actes et prestations mentionnée à l’article L. 162-1-7 du code de la sécurité sociale du test METAglut1™

Specialist prescribes METAglut1™
1: METAglut1™ is prescribed by neuropediatricians, neurologists or geneticists.

Patient Proceeds
with a Blood Draw
2: Blood draw is performed at the hospital, clinic or diagnostic laboratory, in common EDTA vacutainer tubes.
No need for fasting. Samples are stored at 4°C and stored for up to 7 days.
Diagnostic testing lab
performs the test & analysis
3: The hospital, clinic or diagnostic laboratory performs the protocol from the METAglut1™ kit. Samples are processed through flow cytometry.
A report is automatically generated with error detection and/or warnings if any.
A copy of the results is provided to the prescriber.
Results
(24-72 hrs)
Prescription of METAglut1™ in France
Cerba Laboratory and the Bichat Hospital daily perform the METAglut1™ test in France.
Request forms to perform the test in France by those two partners are available down below:

April 2023
Green light and approval from HAS to facilitate reimbursement of METAglut1™ test in France
“METAglut1™ is a valuable addition to the diagnostic arsenal for Glut1DS, and is ascribed a high clinical added value”
(ASA 2)

METAglut1™ is on the market in France, Belgium, and Luxembourg
thanks to our partnership with a leading European Testing Lab, CERBA Laboratoire
First In Vitro diagnostic test
developed from our
METAdiag platform
& using our
proprietary biomarkers

In Vitro Diagnostic Medical Device
to aid the early diagnosis of the
Glut1 Deficiency Syndrome (Glut1DS) a rare neuropediatric disease
(CE marked)

The test has received
funding from the European Union’s Horizon 2020 research and innovation program
(No 806038)
Our latest news on METAglut1™
 February 28, 2025
February 28, 2025Quality management system certification ISO 13485:2016 renewed
 June 20, 2023
June 20, 2023Raising awareness on rare diseases like Glut1DS at the annual meeting of EPNS 2023
 May 3, 2023
May 3, 2023Coverage recommandation for our innovative IVD test METAglut™ by French National Authority for Health (HAS)
 April 27, 2023
April 27, 2023Evaluation of our innovative IVD test METAglut™ published by French National Authority for Health (HAS)
 November 19, 2022
November 19, 2022EIC Accelerator Funding awarded to drive innovation and next-level growth
 September 21, 2022
September 21, 2022Introducing METAflow our innovative AI cytometry data analysis platform at ESCCA 2022
 April 7, 2022
April 7, 2022Testimonial from Dr. Domitille Gras on our innovative IVD test METAglut™ during World Health Day 2022
 February 17, 2022
February 17, 2022Revealing key findings from our METAglut1™ validation study at the meeting of SENP 2022
 February 2, 2022
February 2, 2022Poster presentation on METAglut1™ our innovative IVD blood test at the meeting of SFNP 2022
 September 16, 2021
September 16, 2021Strategic partnership with Sebia to develop innovative in vitro diagnostics
 June 19, 2021
June 19, 2021Latest Findings on our Validation Study Announced at the European GLUT1D Conference
 June 11, 2021
June 11, 2021Updates at the European Conference on Glut1 Deficiency 2021
 March 22, 2021
March 22, 2021Quality management system certification ISO 13485:2016 obtained
 January 15, 2020
January 15, 2020New results on METAglut1™ validation study presented at the meeting of SFNP 2020
 July 11, 2019
July 11, 2019Attending the 2019 Summit of the Glut1 Deficiency Foundation to talk about METAglut1™ our innovative IVD blood test
 June 21, 2019
June 21, 2019Proud Laureate of the PhD Paris Region Funding Initiative
 March 22, 2019
March 22, 2019Oral presentation on METAglut1™ our innovative IVD blood test at the meeting of SENP 2019
 February 20, 2019
February 20, 2019€1M Grant from Bpifrance’s Innovation Prize
 January 16, 2019
January 16, 2019Poster presentation on METAglut1™ our innovative IVD blood test at the meeting of SFNP 2019
 June 22, 2018
June 22, 2018Booth at the European Conference on Glut1 Deficiency 2018
 June 11, 2018
June 11, 2018€3.2M Grant awarded: major boost for the launch of our validation study
 April 27, 2018
April 27, 2018Booth to discuss latest results on METAglut1™ at the meeting of SENP 2018
 July 13, 2017
July 13, 2017Attending the summit of the Glut1 Deficiency Foundation 2017 to talk about METAglut1™ our innovative IVD blood test
 June 12, 2017
June 12, 2017Results of our first study with AP-HP and the ICM on our innovative IVD test METAglut™ published in Annals of Neurology
 February 15, 2017
February 15, 2017European regulatory approval successfully obtained for METAglut1™ our innovative IVD test for Glut1DS
 February 2, 2017
February 2, 2017Launch of our first innovative IVD test METAglut1™ to aid the diagnosis of Glut1DS developed with our partner CERBA HealthCare
 November 18, 2016
November 18, 2016Company milestones shared at the Actionaria Event 2016
 November 7, 2016
November 7, 2016Alliance with Cerba HealthCare to advance metabolomics and drive diagnostic innovations
 October 10, 2016
October 10, 2016Inauguration meeting with our international scientific board of leading experts to enhance GLUT1DS diagnosis
 October 7, 2016
October 7, 2016Attending the first European Conference on Glut1 Deficiency
 April 16, 2016
April 16, 2016Exclusive Interview with BFM Business Radio: our participation in the BigBooster Program
 February 10, 2016
February 10, 2016Selected for the BigBooster Acceleration Program
 July 1, 2015
July 1, 2015